Benzene - Gas Profile



Benzene (C6H6) is a colourless or light yellow liquid at room temperature. It immediately dissipates into the atmosphere. Because its vapour is heavier than air, it may settle in low-lying places. In terms of production volume, it is among the top 20 chemicals and is hugely used in the United States. In several sectors, Benzene is utilised in manufacturing polymers, resins, nylon, and synthetic fibres. It can also make lubricants, rubbers, dyes, detergents, drugs, and pesticides.
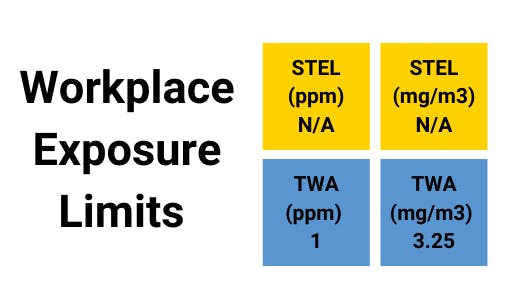
Benzene can be taken into the body through the skin or by eating benzene-containing materials and inhalation. The amount and duration of benzene exposure determine the health consequences on workers. A single exposure to a high concentration (hundreds of ppm) might cause headaches, fatigue, nausea, dizziness, and even unconsciousness if the concentration is extremely high (thousands of ppm), indicating an acute safety event.
Fun Fact – Benzene was utilised as a component in aftershave in the late 19th and early 20th centuries because of its scent.

Applications used in
- Oil and Gas
- Refineries
- HAZMAT