Nitric Oxide - Gas Profile




Nitric Oxide (NO) or nitrogen monoxide is a colourless gas with a sharp, sweet smell. It is made of an oxygen atom and nitrogen atom and is formed by the oxidation of nitrogen. It has the same boiling and melting point - 151.8 Celcius.
Nitric oxide has lots of health benefits for the human body. The human body produces nitric oxide, which is very important for human health. It supports vasodilation, which relaxes the inner muscles of blood vessels, causing them to widen and increase bloody circulation. It is also essential in mammals and acts as a signalling/messenger molecule, helping transmit signals to various parts of the animal autonomy, such as the nervous, immune, and cardiovascular systems.
However, NO is a damaging air pollutant caused by car pollution, automotive engines and thermal power plants. When Nitric Oxide is emitted into the atmosphere, it goes through photochemical reactions with hydrocarbon vapours to form secondary pollutants called 'photochemical oxidants'. These photochemical oxidants create photochemical smog. Nitric oxide is a compound formed during the oxidation of ammonia to nitric acid. Nitric oxide, combined with water vapour in the atmosphere, forms nitric acid, one of the main components of acid rain.
Fun Fact – Deficiencies of nitric oxide has been found to impact the memory and learning of humans and causes the development of diseases such as Alzheimer's and Huntington's

Applications used in
- Oil and Gas
- Laboratories
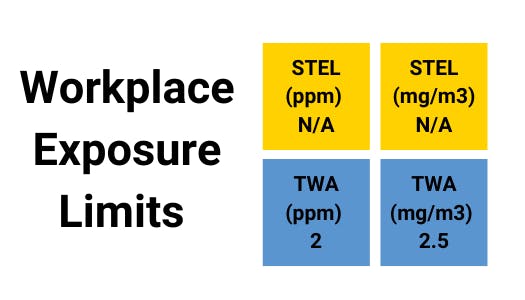
Assessment of the Dangerous Toxic Load (DTL) for Specified Level of Toxicity (SLOT) and Significant Likelihood of Death (SLOD)
- CAS number – 10102-43-9
- 'n' value - 1
- SLOT DTL (ppmn.min) – 2.09 x 10
- SLOD DTL (ppmn.min) – 2.43 x 10




