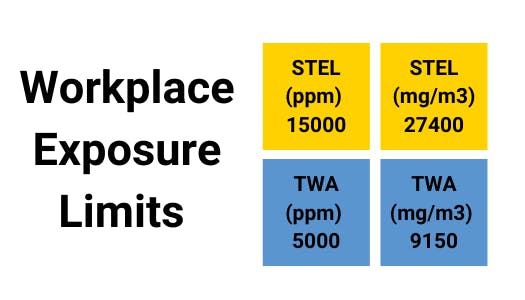
Carbon Dioxide - Gas Profile



Carbon dioxide (CO2) is a colourless gas with a sour taste and a mild harsh odour. It is one of the most important greenhouse gases linked to global warming, but it is a minor component of the Earth's atmosphere (about 3 volumes in 10,000), formed in the combustion of carbon-containing materials, fermentation, and animal respiration, and used by plants in carbohydrate photosynthesis.
Jan Baptista van Helmont, a Belgian scientist, identified carbon dioxide as a distinct gas early in the 17th century after observing it as a result of both fermentation and combustion. At 31 °C (87.4 °F), it liquefies to 75 kilogrammes per square centimetre (1,071 pounds per square inch), or 16–24 kg per sq cm (230–345 lb per sq in.) at 23–12 °C (10–10 °F). Carbon dioxide is used as a refrigerant, to inflate life rafts and life jackets, to blast coal, to foam rubber and plastics, to promote plant growth in greenhouses, to immobilise animals before slaughter, and to make carbonated beverages.
Fun Fact – Carbon Dioxide is used to dispense beer

Applications used in
- Chemical Production
- Refrigerant
- Agriculture
- Food and Beverage