Chlorine - Gas Profile




Chlorine (Cl2) is a gas that appears green/yellow and has a pungent, suffocating smell associated to the smell of bleach. Chlorine is in the ‘halogens’ gas family, alongside fluorine, bromine, and iodine. It is a toxic and corrosive gas that can irritate the human eyes and respiratory system. Chlorine doesn’t burn but supports combustion, similar to oxygen.
The creation of Chlorine dates back to the presence of rock salts (halogen salts) thousands of years ago, the main constituent of the salt dissolved in seawater. In Roman times, a chemist named Johann Rudolf Glauber, heated salt in a charcoal furnace and condensed the fumes to create a gas. This is a gas we know now as hydrochloric acid. After discovering hydrochloric acid, another chemist named Carl Wilhelm Scheele combined the newly found gas with black oxide with manganese and created a green/yellow gas. Although found by Carl, it was Humphry Davy - an English chemist who later named the discovery of the gas - Chlorine.
- Chlorine exists naturally in small amounts within volcanic gases in the form of chemical compounds.
- The most common Chlorine compound is sodium chloride which is found in crystalline rock salt in seawater - predominantly the dead sea and salt lakes.
- Chlorine can be found in small quantities in the human body in our bloodstream.
- Chlorine becomes a liquid at -34 degrees.
- Chlorine is most known for its use in swimming pools, here it is used to sterilise water to protect humans from cholera and typhoid.
Fun Fact – The name ‘chlorine’ is derived from the Greek word ‘chloros’, meaning ‘yellowish green’.

Applications used in
- In rock salt deposits
- Laboratories
- Swimming pools
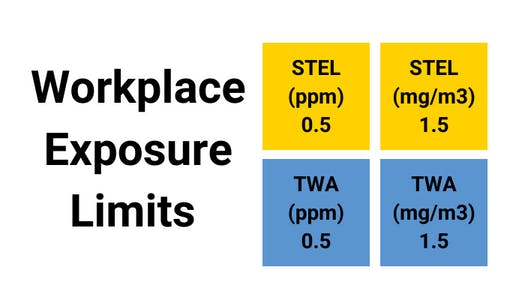
Assessment of the Dangerous Toxic Load (DTL) for Specified Level of Toxicity (SLOT) and Significant Likelihood of Death (SLOD)
- CAS number - 7782-50-5
- 'n' value - 2
- SLOT DTL (ppmn.min) - 1.08 x 105
- SLOD DTL (ppmn.min) - 4.84 x 105





