Chlorine Dioxide - Gas Profile




Chlorine Dioxide (ClO2) has a greenish-yellow colour and unpleasant odour similar to Chlorine. Chlorine Dioxide's chemical structure and behaviour are drastically different from elemental chlorine. Chlorine Dioxide is an extremely powerful but highly tiny and volatile chemical. Chlorine Dioxide is a free radical in dilute water solutions and has a vigorous reaction with reducing agents at high doses.
This compound was first identified by Sir Humphrey Davy in 1814 and is known today as chlorine dioxide. His gas was created by reacting potassium chlorate with sulphuric acid (H2SO4) (KClO3). Then he used hypochlorous acid instead of sulphuric acid (HOCl). Recently, this process has also been utilised to mass-produce chlorine dioxide. Short-term Chlorine dioxide can cause respiratory discomfort and damage the respiratory tract if inhaled. The start of symptoms like coughing, wheezing, and severe breathing difficulties may be prolonged.
Other facts to know about the gas are:
- Chlorine Dioxide has a melting point of -59 degrees celsius and a boiling point of 11 degrees Celsius.
- It is denser than air and is water soluble at standard temperatures and pressures up to 250ppm.
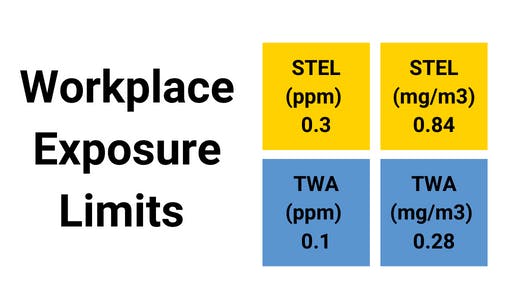
- It has been shown that concentrations of chlorine dioxide as low as five parts per million are safe for application on food-contact surfaces (ppm).
- Chlorine dioxide donates oxygen and decomposes into water, oxygen, and ordinary table salt, while chlorine bleach and bromide create carcinogenic trihalomethanes that are then rinsed down the drain and deposited in the environment. As a result, ClO2 is significantly safer for machinery and a better option for the planet.
Fun Fact – Chlorine Dioxide in low concentrations is effective in reducing E. coli that is present in water.

Applications used in
- Food preparation & packaging
- Paper production
- Water treatment




