Phosphine - Gas Profile



Phosphine (PH3), commonly known as hydrogen phosphide, is a colourless, flammable, highly poisonous gas with a garlic-like odour. Although phosphine is structurally identical to ammonia (NH3), it is a considerably worse solvent and is much less water-soluble.
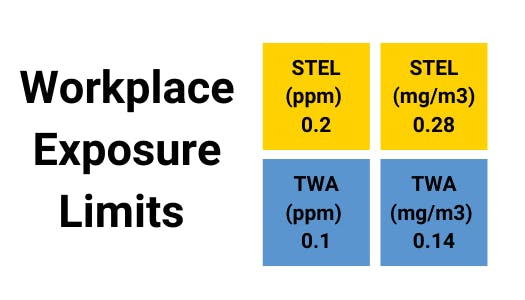
When a strong base or hot water reacts with white phosphorus, or when water reacts with calcium phosphide (Ca3P2), phosphine is generated. Phosphine (PH3) has a boiling point of -126°F and a freezing point of -209°F. In extremely low quantities, it is extremely poisonous when inhaled. Additionally, long-term heating could cause containers to explode violently, resulting in possible injuries or fatalities. Workers exposed to phosphine on a long-term basis may have nasal and throat irritation, weakness, dizziness, nausea, gastrointestinal, cardiorespiratory, central nervous system symptoms, jaundice, liver effects, and enhanced bone density.
Fun Fact – Phosphine often gives off a fishy aroma

Applications used in
- Agriculture
- Electronics Industry
- Laboratories
- Pest Control